Story
In focus: Ocean Acidification
1 November 2023

Above: Crustaceans are one of the many types of organism at increased risk from ocean acidification.
You might remember the 'pH scale’ from Chemistry lessons at school, which is used to measure the acidity or alkalinity of a liquid. The lower the pH value of a solution, the higher the acidity. Whereas solutions with higher pH values are more alkaline.
.jpg)
The term ‘Ocean Acidification’ was coined in 2003, and it is used to describe the ongoing decrease in ocean pH – the ‘acidification’ of our ocean.
What is causing ocean acidification?
The primary cause of ocean acidification is the increasing concentration of carbon dioxide (CO2) in the Earth's atmosphere, driven by human activities. When we burn fossil fuels like coal, oil, and natural gas – carbon dioxide enters the atmosphere. But it doesn’t stay there. Our Director of Science Professor Steve Widdicombe explains:
“Around 25% of all the carbon dioxide that humans produce every year - that we send into the atmosphere - ends up in the ocean. And it then reacts with the seawater and dissolves, and in doing so changes the chemistry; it reacts with water molecules to create a weak acid called carbonic acid - which is the chemical that is driving this phenomena called ocean acidification.”
“The ocean has been doing its very best to help us. The absorption of carbon dioxide into the ocean is actually part of a natural process in which carbon cycles around the planet - from the geology into the atmosphere, back into the oceans, and then creating rocks again at the bottom of the sea.”
“But what is happening now is that the ocean can’t do it quickly enough at the rate at which humans are putting carbon dioxide into the atmosphere."
“Ocean acidification is happening in all the world’s oceans, and the oceans are acidifying at a much faster rate than they were 20 years ago."
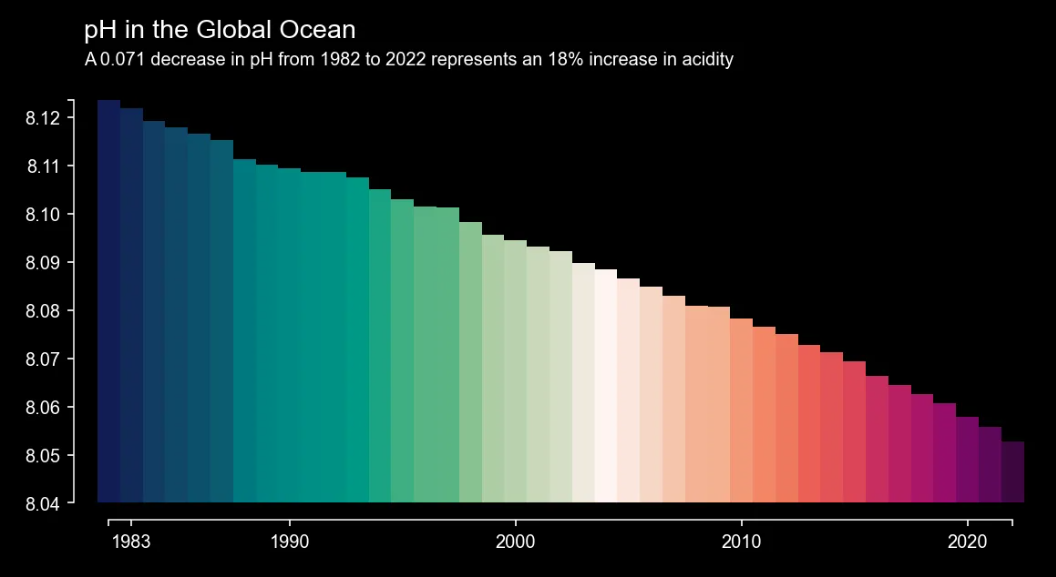
Above: The pH value of the global ocean decreased by 0.071 units from 1982 to 2021. Since the pH value is on a logarithmic scale, this corresponds to an increase in acidity of 18%. [Source]
What are the effects of ocean acidification?
The increasing acidity of our ocean has widespread and severe implications for marine ecosystems.
Ocean acidification changes the ease at which calcium carbonate minerals are made and dissolved. Calcium carbonate is vital to many marine organisms that use it to build their skeletons or shell structures – such as corals, shellfish and crustaceans, and several important groups of plankton – which play an essential part in the wider marine food web.
As a result, these organisms are at an increased risk from ocean acidification, with species being impacted already. If the pH gets too low, shells and skeletons begin to dissolve.
Professor Widdicombe adds: “This is hugely significant for ecosystems, the wider marine food web, and indeed, our own food security.”
Furthermore, due to the unprecedented rate of acidification, these vulnerable organisms may not have time to evolve mechanisms to cope with the changing chemistry of the ocean.
One of our key ocean acidification research findings has been that the impact of ocean acidification on marine species is also strongly dependent on the interaction with other stressors associated with global change, such as warming.
.png) Above: Dying coral reef. Corals are one of the many marine organisms at increased risk from ocean acidification.
Above: Dying coral reef. Corals are one of the many marine organisms at increased risk from ocean acidification.
What can we do to mitigate ocean acidification?
Professor Widdicombe addresses the need for urgent action:
“So, what do we do? Well, first we need to stop emitting such vast quantities of carbon dioxide into the atmosphere. That’s our first step to trying to address this problem.”
“But, there are other things we can also do. There are natural processes in natural habitats - things we call ‘Blue Carbon’ habitats. So: seagrasses, and mangroves, and saltmarshes, which trap carbon dioxide from the atmosphere and lock carbon away into the into the sediment. So, they sequester that carbon for us. So, it’s important that we protect and restore those habitats and allow them to do those natural processes.”
“There are other processes that work in the open ocean where phytoplankton ‘fix’ the carbon dioxide that’s available and turn it into organic matter, and that filters down into the deep ocean. Again, we need to make sure we keep and maintain a healthy ocean ecosystem to allow them to do that.”
“And then finally, there’s adaptation and resilience, we need to create human communities that are resilient and that can cope with the changes that are coming.”
.png) Above: 'Blue carbon' habitats like mangroves have the ability to store huge quantities of carbon, and these nature-based solutions are a vital component in efforts to mitigate ocean acidification.
Above: 'Blue carbon' habitats like mangroves have the ability to store huge quantities of carbon, and these nature-based solutions are a vital component in efforts to mitigate ocean acidification.
Related information
PML’s work on ocean acidification
Ocean Acidification Research for Sustainability (OARS) programme
A global framework for ocean acidification
PML’s ocean acidification work shortlisted for the 2023 NERC Impact Awards
PML funded to become Secretariat for the UN-endorsed 'OARS' programme
OSPAR report on ocean acidification
Global Ocean Acidification Observing Network (GOA-ON)